Establishing expiry date for clinical diagnostic reagents
By A Mystery Man Writer
Last updated 19 May 2024

Product shelf life is an essential product performance requirement that, along with other design requirements, is used to determine the safety and efficacy of a clinical diagnostic
Shelf life - Wikipedia

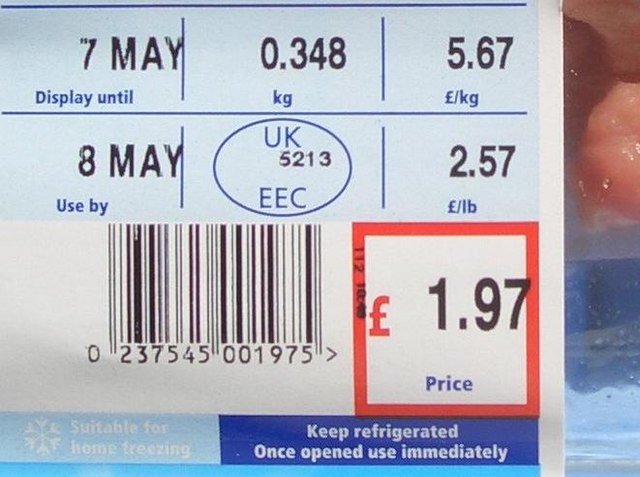
Alcohol urine test

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF

ASTM D7160-16 Red - Standard Practice for Determination of

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF

INDICAID PoC (Professional) COVID-19 RAPID ANTIGEN EXTERNAL

ASTM F2477-23 - Standard Test Methods for in

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF

How to Handle Lab Reagents After Their Expiration Date
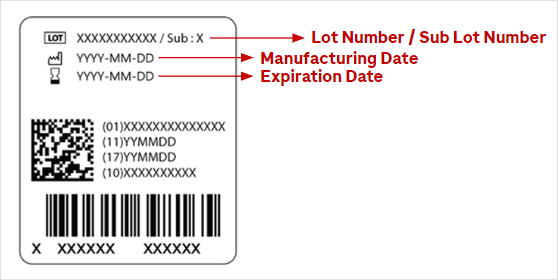
Pilot COVID-19 At-Home Test

What Is Product Development? 7 Steps to Making a Product (2024

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF
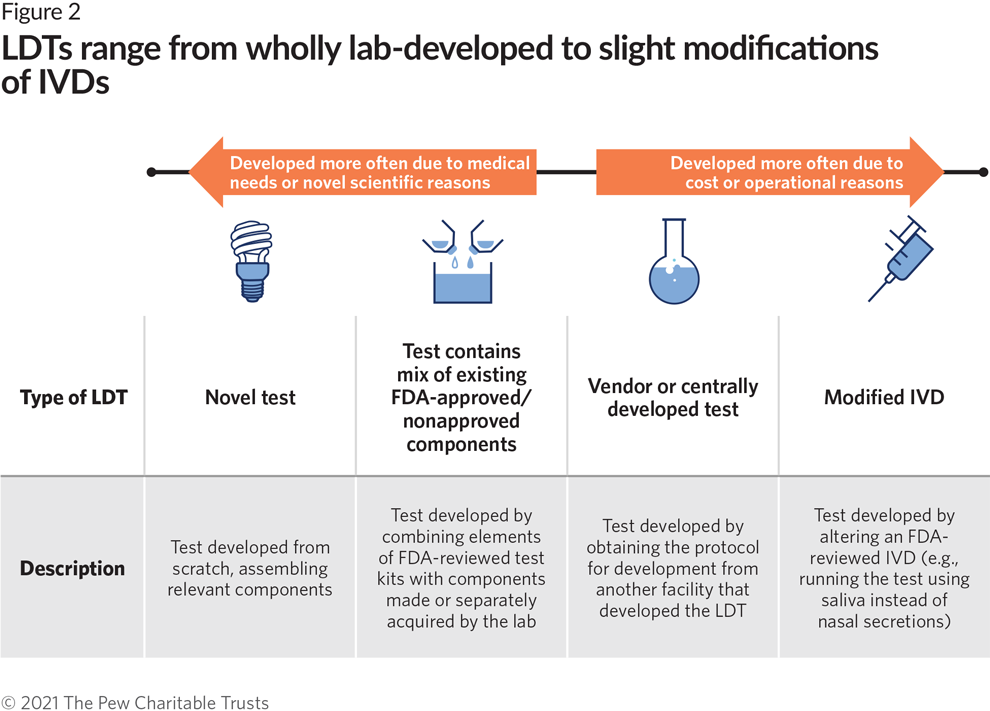
Diagnostic Tests Not Reviewed by FDA Present Growing Risks to
Recommended for you
 What's The Difference: Best Before vs. Expiry Date14 Jul 2023
What's The Difference: Best Before vs. Expiry Date14 Jul 2023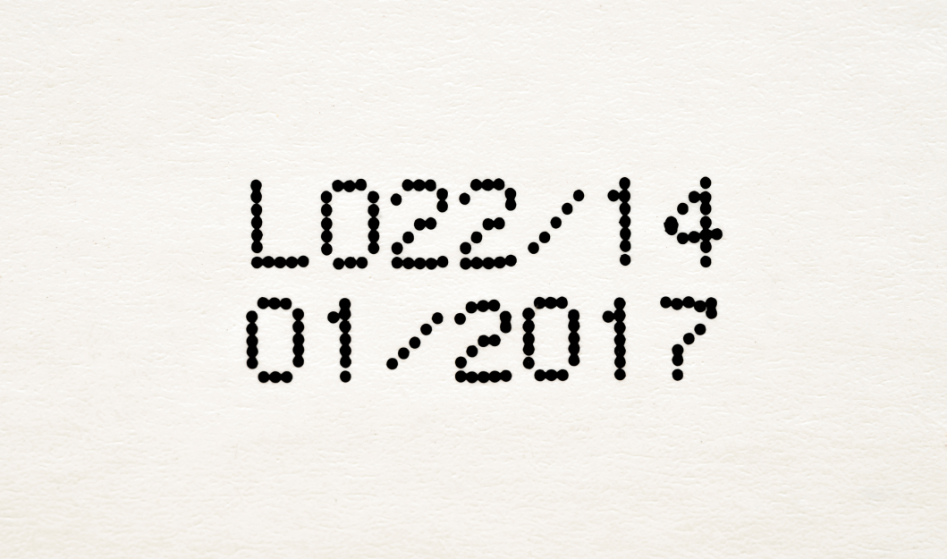 Medication Expiration Date Guidelines by FDA14 Jul 2023
Medication Expiration Date Guidelines by FDA14 Jul 2023 What does a medicine's expiration date mean?14 Jul 2023
What does a medicine's expiration date mean?14 Jul 2023 Does Butter Expire—and How Can You Tell If It's Gone Bad?14 Jul 2023
Does Butter Expire—and How Can You Tell If It's Gone Bad?14 Jul 2023 Understanding The Expiration Date of Chocolate, Cocoa Box14 Jul 2023
Understanding The Expiration Date of Chocolate, Cocoa Box14 Jul 2023 Medicine expiry dates - Dr Fox14 Jul 2023
Medicine expiry dates - Dr Fox14 Jul 2023 Can Perfume Expire?14 Jul 2023
Can Perfume Expire?14 Jul 2023- How to find out M&M's expiration date - Quora14 Jul 2023
 Expiry Dates, Best Before Dates and Date Codes – Wildly Delicious14 Jul 2023
Expiry Dates, Best Before Dates and Date Codes – Wildly Delicious14 Jul 2023 High Court Issues Notice To Centre, Delhi Govt In Suo Moto PIL14 Jul 2023
High Court Issues Notice To Centre, Delhi Govt In Suo Moto PIL14 Jul 2023
You may also like
 Sequins Crop Top - Aureliana14 Jul 2023
Sequins Crop Top - Aureliana14 Jul 2023 Plus sized models don't have big bellies. Have you noticed?14 Jul 2023
Plus sized models don't have big bellies. Have you noticed?14 Jul 2023 Monvecle Baby Cotton Warm Vests Unisex Infant to Toddler Light Padded Waistcoat Brown Rabbit 3-6m : : Clothing, Shoes & Accessories14 Jul 2023
Monvecle Baby Cotton Warm Vests Unisex Infant to Toddler Light Padded Waistcoat Brown Rabbit 3-6m : : Clothing, Shoes & Accessories14 Jul 2023 Tiny Bunny Florals Ditsy Flowers Fabric by Timeless Treasures - modeS4u14 Jul 2023
Tiny Bunny Florals Ditsy Flowers Fabric by Timeless Treasures - modeS4u14 Jul 2023 CYPRESS White Knee High Platform Boots | Women's Designer Boots – Steve Madden Canada14 Jul 2023
CYPRESS White Knee High Platform Boots | Women's Designer Boots – Steve Madden Canada14 Jul 2023 Greca Border Briefs14 Jul 2023
Greca Border Briefs14 Jul 2023 Baby-Girl-White-Deep-Lace-Frilly-Socks14 Jul 2023
Baby-Girl-White-Deep-Lace-Frilly-Socks14 Jul 2023 Terra & Sky Women's Plus Size Flare Leggings, 2-Pack14 Jul 2023
Terra & Sky Women's Plus Size Flare Leggings, 2-Pack14 Jul 2023 Chantelle HIGH WAIST BRIEFS - Briefs - black - Zalando.de14 Jul 2023
Chantelle HIGH WAIST BRIEFS - Briefs - black - Zalando.de14 Jul 2023- 7 In 1 Travel Organizer Travel Pouch Travel Luggage Bag Pouch Organizer Storage Foldable Waterproof For Puka LIving Store14 Jul 2023
